Ever wondered what makes your favorite Sprite fizzy and tangy? The answer lies in its unique composition, which includes carbonic acid and citric acid. Through this comprehensive guide, you’ll delve deep into the chemistry of Sprite to figure out whether it’s an acid or a base.
Ready to unlock the secrets behind your go-to soft drink’s pH level? Let’s dive right in!
You Are Watching: Is Sprite An Acid Or Base Updated 07/2025
Exploring The Chemistry Of Sprite

Sprite is a carbonated soft drink that contains carbonic acid and citric acid as its main ingredients.
Carbonic Acid In Carbonated Soft Drinks
Sprite, like other carbonated soft drinks, contains a key ingredient known as carbonic acid. This compound is infused into the drink under pressure, giving Sprite its signature fizziness and tangy taste profile.
Despite what the term “acid” might suggest, carbonic acid is actually classified as a weak acid. It’s this specific characteristic that lends itself to maintaining the desirable attributes of Sprite without making it overly acidic or corrosive.
This unique balance between acidity and basicity in Sprite comes from how carbonic acid behaves within its mixture. When we take a sip of our beloved lemon-lime flavored soft drink first introduced back in 1961, we experience the pleasantly mild burn associated with bubbles popping — all thanks to the gentle release of CO2 from H2CO3 (carbonic acid).
The fact that Sprite has managed to harness such an intricate chemical composition while still boasting a slightly higher pH level than most other sodas indeed makes it an exemplary player in the fizzy world!
Citric Acid As The Main Ingredient In Sprite
Read More : How Many Calories In Pink Whitney Updated 07/2025
Sprite contains citric acid as its main ingredient. Citric acid is a natural compound found in citrus fruits such as lemons and limes. It gives Sprite its tangy and refreshing flavor. Citric acid is added to many soft drinks, including Sprite, not only for taste but also as a preservative and to enhance the shelf life of the beverage.
This weak acid plays a crucial role in balancing the pH level of carbonated soft drinks like Sprite, helping to create a pleasant drinking experience while maintaining freshness.
Determining The PH Of Sprite

Comparing the pH levels of Sprite to identify its acidity or basicity is essential.
Comparing PH Levels To Identify Acidity Or Basicity
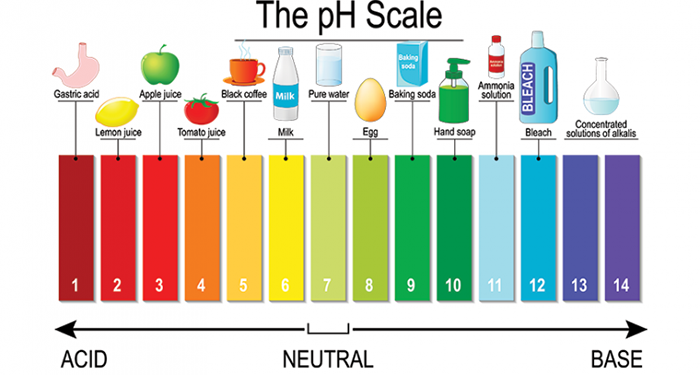
Comparing pH levels is an effective way to determine the acidity or basicity of a substance. In the case of Sprite, its pH level plays a crucial role in understanding its chemical properties and effects. Here’s what you need to know:
- Acids have a pH level below 7, indicating high acidity, while bases have a pH level above 7, showing basicity.
- Carbonated soft drinks like Sprite contain carbonic acid (H2CO3) under pressure. While carbonic acid is considered a weak acid, it contributes to the beverage’s refreshing carbonation.
- Sprite’s pH level is measured at 3.29, making it slightly acidic compared to other sodas.
- Understanding the pH scale helps identify whether a substance is more acidic or basic. For example, vinegar has a lower pH value than water and is therefore more acidic.
- Other common substances that can be used for comparison include soap (basic), baking soda (basic), milk of magnesia (basic), and lemon juice (acidic).
PH Value Of Sprite And Its Significance
In this case, Sprite falls on the slightly acidic side of the scale with a pH level of 3.29.
Understanding the significance of Sprite’s pH level helps us grasp its potential impact on our bodies. Acidity levels can affect tooth enamel, as acidic beverages have been linked to tooth erosion over time.
While Sprite is considered a healthier choice compared to other sodas due to its relatively lower acidity, it’s still important to consume such drinks in moderation and maintain good oral hygiene practices like rinsing with water after consumption.
Is Sprite An Acid Or A Base?

To determine if Sprite is an acid or a base, we need to analyze its pH level and compare it to the standard pH ranges for acidity and basicity.
Defining Acids And Bases
However, unlike other sodas that tend to be highly acidic, Sprite is considered less harmful to your teeth due to its slightly higher pH level of 3.29. This makes it fall on the more neutral side compared to other acidic beverages.
So even though it may contain certain acids like citric acid and carbonic acid, Sprite can still be enjoyed without causing significant harm to your overall health when consumed in moderation.
Analyzing The PH Level Of Sprite To Determine Its Classification
- The pH scale measures the acidity or basicity of a substance, with acids having a pH below 7 and bases having a pH above 7.
- Sprite has a pH level of 3.29, which classifies it as slightly acidic compared to other sodas.
- Despite being slightly acidic, Sprite is still considered a healthier choice compared to other sodas due to its lower sugar content.
- Carbonic acid, which gives Sprite its carbonation, is a weak acid and contributes to the overall acidity of the beverage.
- Consuming acidic drinks like Sprite in moderation is generally safe for individuals without pre – existing health conditions.
- Acids can be corrosive and react with other substances, so it’s important to consider the pH level when understanding their potential effects.
It’s interesting to note that even though Sprite contains citric acid as its main ingredient and carbonic acid for carbonation, the overall pH level of the drink places it in the base category.
This demonstrates that not all products containing acids are strictly classified as acids themselves.
It’s important to remember that everything should be consumed in moderation, and maintaining a balanced diet is crucial for overall health.
Conclusion
Although it contains carbonic acid under pressure, Sprite is considered a weak acid with a pH level of 3.29, making it slightly acidic compared to other sodas.
Understanding the acidity or basicity of beverages like Sprite can help us make informed choices about our consumption and better understand their chemical properties.
Sources: https://chesbrewco.com
Category: Drink