Ever pondered the question, “Do all liquids weigh the same?” Well, contrary to what many may assume, each liquid carries a distinct weight.
Our deep dive into this intriguing topic will explore the reasons for these variances and illuminate how understanding liquid density can alter your perspective on everyday substances.
You Are Watching: Do All Liquids Weigh The Same Updated 07/2025
Get ready to have your mind blown by liquefied science!
Understanding Liquid Density

What is density?
Density, in simple terms, is about how tightly packed the particles within a substance are. Consider it as a measure of ‘compactness’. In relation to liquids, density refers to the amount of mass a liquid possesses in a particular volume.
For instance, if you take equal volumes of oil and water, they won’t weigh the same. This difference arises due to their varying densities – water has more mass packed into its given volume, hence its greater weight per unit compared to oil.
It’s essential then, when discussing weights of different liquids like alcohol or coffee versus water or milk that we bring up density into our conversation because this ultimately determines the weight variations we observe among these fluids.
The relationship between volume and weight
The weight of a liquid is influenced by its volume. Volume refers to the amount of space that a liquid takes up, while weight is the force exerted on an object due to gravity. When comparing two liquids, if they have the same volume, the liquid with higher density will weigh more.
Density is a measure of how tightly packed the molecules are in a substance. So, even though two liquids may occupy the same amount of space, their weights can differ because of variations in their densities.
For example, let’s consider water and oil. Water has a higher density than oil, which means it has more mass per unit volume compared to oil. If we take equal volumes or amounts of water and oil, we will find that water weighs more than oil due to its higher density.
This relationship between volume and weight highlights that not all liquids weigh the same – their densities play a significant role in determining their weights.
It’s important to note that understanding this relationship can help us comprehend why different liquids have varying weights and why they behave differently when mixed together or when subjected to external forces like gravity or buoyancy.
Do All Liquids Weigh the Same?
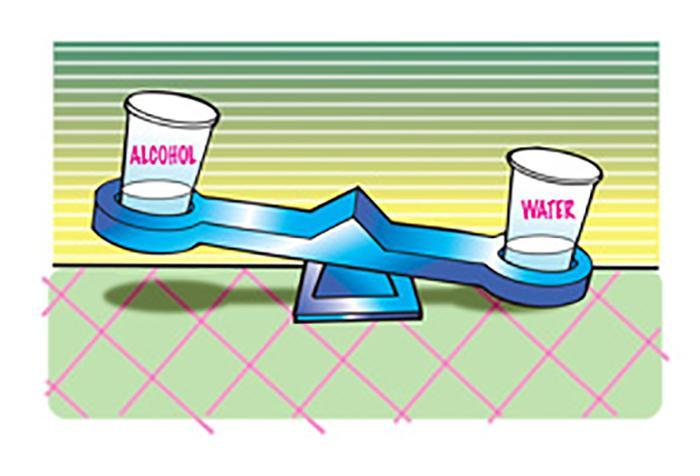
Exploring different liquid densities
Liquid densities vary based on factors such as temperature, pressure, and composition. Here are some key points to consider when exploring different liquid densities:
- Different liquids have varying densities because they contain different types and amounts of particles.
- The density of a liquid is determined by the mass of its particles and the volume it occupies.
- Liquids with more densely packed particles will have a higher density, resulting in a greater weight for an equal volume compared to liquids with less dense particles.
- Density is often expressed in units such as grams per milliliter (g/mL) or pounds per gallon (lbs/gal).
- Water is often used as a reference point for comparing the density of other liquids. It has a density of 1 g/mL or approximately 8.34 lbs/gal.
- Some liquids, like mercury or lead, have much higher densities than water and therefore weigh more for the same volume.
- On the other hand, lighter liquids such as alcohol or gasoline have lower densities and weigh less for the same volume compared to water.
- The difference in liquid densities also affects their behavior when mixed. For example, oil floats on top of water because it has a lower density.
Comparing the weight of water to other liquids
While many of us may assume that all liquids have equal weight, this belief is actually debunked when we delve into the concept of liquid density.
To illustrate, let’s compare the weight of water to other common liquids such as olive oil, ethanol, and honey.
The following HTML table puts it all in perspective:
| Liquid | Density (g/cm³) | Comparison with Water |
|---|---|---|
| Water | 1 | Baseline |
| Olive Oil | 0.92 | Lighter |
| Ethanol (Alcohol) | 0.789 | Significantly Lighter |
| Honey | 1.42 | Heavier |
From the table, it’s clear that not all liquids have the same weight. Water, usually considered as the standard, has a density of 1 g/cm³.
Olive oil and ethanol, both popular alcoholic beverages, have densities lower than water, making them lighter.
In contrast, honey, revered for its dense texture and rich flavor, has a higher density than water, making it the heavier liquid. In essence, the weight of a liquid is undeniably tied to its density.
Factors that affect liquid density
- Temperature: The temperature of a liquid can significantly affect its density. As the temperature increases, the particles in the liquid gain more energy and spread out, resulting in a decrease in density. Conversely, as the temperature decreases, the particles move closer together, increasing the density of the liquid.
- Pressure: Changes in pressure can also impact the density of a liquid. When pressure increases, the volume of a liquid decreases, leading to an increase in density. On the other hand, when pressure decreases, the volume expands, resulting in a decrease in density.
- Impurities and solutes: Adding impurities or solutes to a liquid can alter its density. For example, when salt is dissolved in water, it increases the density of water due to the added mass from salt molecules.
- Composition and molecular structure: The composition and molecular structure of a liquid play a significant role in determining its density. Different substances have different atomic masses and arrangements, which directly impact their densities. For instance, oil has a lower density than water because its molecules are less tightly packed.
- Dissolved gases: Gases dissolved within liquids can affect their densities. For instance, carbonated beverages have higher densities due to the presence of dissolved carbon dioxide gas.
By understanding these factors that influence liquid density, we can better comprehend why not all liquids weigh the same.
Temperature changes, pressure variations, impurities/solutes content, composition/molecular structure differences, and dissolved gases all contribute to varying densities among liquids.
It’s crucial for alcoholics to be aware of these factors as they navigate their relationship with various alcoholic beverages that may have different densities and weights.
Examples of Liquids with Different Weights

Read More : Can Red Bull Break Glass Updated 07/2025
– Mercury, a liquid metal with a high density, is one of the heaviest liquids on Earth.
– In contrast, lighter liquids like alcohol and oil have lower densities and weigh less than water.
– Wine, coffee, and milk also have different weights compared to water due to their varying densities.
Heaviest and lightest liquids on Earth
There are some liquids that are heavier or lighter than others, and it all comes down to their densities. Density is a measure of how heavy a liquid is in relation to its volume.
The heaviest known liquid on Earth is mercury, which has a density of about 13.5 times that of water.
On the other end of the spectrum, the lightest liquid is hydrogen gas, which has an extremely low density compared to most other liquids. So while not all liquids weigh the same, they can vary significantly depending on their density.
Comparing the weights of wine, alcohol, coffee, and milk to water
Wine, alcohol, coffee, and milk are all liquids that have different weights when compared to water. This is because each of these liquids has a unique density, which affects their weight.
For example, wine is generally heavier than water because it has a higher density due to the presence of sugars and other components.
Alcohol also tends to be lighter than water because it has a lower density. Coffee can vary in weight depending on its concentration and additives like cream or sugar.
Milk is slightly denser than water due to the proteins and fats it contains. So, when comparing the weights of these liquids to water, it’s important to consider their respective densities as well.
Conclusion
Understanding liquid density is important because it helps us comprehend why different liquids have varying weights. Contrary to popular belief, not all liquids weigh the same. The density of a liquid plays a crucial role in determining its weight and how it compares to other liquids.
By grasping this concept, we can better appreciate the differences between various fluids and their weights.
Sources: https://chesbrewco.com
Category: Drink